Are you an importer of cosmetics in India or willing to be then you are on the right platform. Here you will get to know about how you can simply enter in market with the license and which documents required for cosmetics import license. Because, cosmetics market is growing rapidly and people are willing to use trusted products.
So, to stand out in the market you must obtain the license first. This will increase your credibility and ensure that you comply with all the cosmetics regulatory rules.
Go with the below-mentioned guide before jumping into the cosmetics import business in India.
What is a Cosmetics Import License?
A cosmetic import certification is a legal document issued by the regulatory authority that allows businesses to import cosmetics legally into the nation. It ensures that the imported cosmetics meet the safety, quality, and cosmetic labeling standards being marketed.
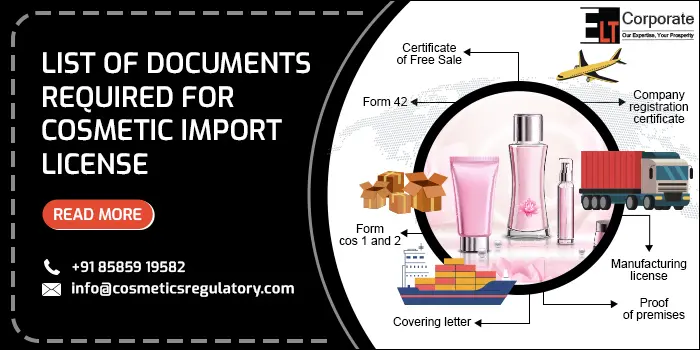
Documents Required For Cosmetics Import License In India
When you apply for the import license of cosmetics then you must understand the documents required for a cosmetics import license. If you omit any of the documents then the license application will be rejected and you have to apply for it again. So to avoid these mistakes you must apply for the cosmetics import license in India under the expert’s guidance with the following documents:
- Cover Letter: This is the primary document for getting an Import License for cosmetics. It acts as a guidebook for the reviewer it contains the purpose of the form, product information file (PIF), and all the documents list
- COS-1 Application Form: The COS-1 Form is the registration form for the Import of cosmetics. It contains all the details about the product and manufacturers. All the details must be filled in carefully to avoid any delay
- Power of Attorney Certificate: This certificate is submitted in case of foreign manufacturers and it must be notarized
- Free Sale Certificate: This certificate is granted by the regulatory body of the manufacturer’s company. It Confirms that the product is sold freely in the manufacturing company and is of standard quality, safe, and effective for customer use
- List of Ingredients: This contains the list of all the ingredients used in the manufacturing of cosmetics. All the ingredients must be of good quality grade and don’t cause any harmful side effects
- Non-Animal Testing Declaration: Any product tested on animals is banned from import in India from 12th November 2014. An undertaking is submitted by Manufacturers as well as importers
- Labelling Details: Both the inner and outer contain all the essential details about the product and manufacturers like the name of the product, manufacturer details, batch number, manufacturing and expiry date, ingredients with their composition, warnings, and instructions
- Declaration of Hexachlorophene and Heavy metals: The declaration must be submitted by the manufacturers stating that the cosmetic products contain heavy metals( Arsenic)and Hexachlorophene in the specified limit as per cosmetic rule, 2020
- Presence of colored additives and ingredients like Lead and Mercury: The presence of colored additives in cosmetic products leads to additional testing data for regulatory approval.
- Leaflet: It contains instructions like warnings, storage conditions, and usage guidelines. It helps a consumer to use them safely and effectively.
Why Do You Need Cosmetic Import Certification?
A Cosmetic Import Certification is necessary for companies that want to import and sell cosmetics legally in a nation. It guarantees that products are safe, of good quality, and compliant with regulations before they hit the market. Here’s why this certification is important:
1. Compliance with Regulations
Most countries have strict regulations on the importation of cosmetics. Certification ensures that your products are compliant with:
- Ingredient safety standards (e.g., no prohibited or toxic chemicals).
- Proper packaging and labeling (according to local laws).
- Good Manufacturing Practices (GMP) for product quality control.
2. Consumer Safety and Confidence
- Prevents contaminated or unsafe products from reaching the market.
- Ensures disclosure of ingredients and allergens.
- Creates consumer confidence in your brand by showing adherence to global safety standards.
3. Evading Legal Problems & Import Delays
- Without certification, customs officials can seize, reject, or hold up shipments.
- Non-adherence can lead to fines, penalties, or import bans.
- Certification streamlines the customs clearance process, avoiding unnecessary delays.
4. Market Access & Business Expansion
- Numerous retailers and e-commerce websites demand certification proof before listing products.
- Certification enables brands to grow internationally without legal issues.
- Adherence to key regulatory agencies (e.g., FDA, EMA, CDSCO, NMPA) facilitates global expansion.
5. Competitive Advantage
- Certified products are perceived as more trustworthy and high-quality.
- Certification can also be employed as a marketing instrument to lure consumers who seek safe and legally sanctioned products
Who Needs to Apply for a Cosmetics Import License?
Any business that wants to import cosmetics into a country must apply for a cosmetics import license to ensure regulatory approval. This typically includes:
- Manufacturers with International Sourcing: Cosmetic companies importing ingredients or finished products from other countries.
- Pharmacies & Beauty Stores: If importing directly from international brands.
- Cosmetic Importers & Distributors: Businesses that import cosmetics for resale or distribution.
- Retailers & Wholesalers: Companies that sell imported cosmetics to consumers or other businesses.
- E-commerce Sellers: Online sellers importing cosmetics from international brands.
When Should You Start the Process of Obtaining the License?
You need to initiate the process of acquiring a cosmetics import license in advance usually 3 to 6 months before importing. The timeline may vary based on the regulations of the country and the complexity of the approval process.
What Is the Importance Of Cosmetic Import License?
Before applying for a cosmetic Import license, you must know the need and importance of it. The various importance are as follows:
- For delivering good quality, safe, and standard products to consumers
- Build customer trust in the brand
- Enhance Brand reputation and sales
- Maintain Regulatory compliance of cosmetic products as per regulatory requirements
- Ban the sale of Misbranded, spurious, or Adulterated cosmetic products contributing to customers’ safety.
Step-by-Step Process For Cosmetic Import License
Importers or manufacturers of cosmetic products can apply for a cosmetic Import License by following the below steps:
Identify Importer & Manufacturer
- The manufacturer, importer, or authorized agent can apply.
- Foreign manufacturers must appoint an Indian representative.
Register on CDSCO’s SUGAM Portal
- Create an account at CDSCO SUGAM.
Prepare Required Documents
- Form COS-1 (Application form)
- Covering Letter to DCGI
- Power of Attorney (PoA)
- Cosmetic Manufacturing License (from the home country)
- Free Sale Certificate (FSC)
- Ingredient List & Cosmetic Labeling Compliance
- Product Specifications & Safety Data
- Certificate of Analysis (CoA)
- Good Manufacturing Practice (GMP) Certificate
Submit Application & Pay Fees
- Upload documents on the CDSCO SUGAM portal.
- Pay the prescribed government fee.
CDSCO Review & Queries
- CDSCO reviews the application and may request clarifications.
Issuance of Import Registration Certificate
- If approved, CDSCO grants the Import Registration Certificate (Form COS-2) (valid for three years).
Import & Regulatory Compliance
- Start importing and ensure compliance with labeling and safety regulations.
Where Should You Submit the Documents?
All applications and the documents required for a cosmetics license in India are submitted to the CDSCO (Central Drugs Standard Control Organization). It is operated under the Ministry of Health & Family Welfare, Government in India. You have two options to apply whether you can apply through an online portal or physically submit the documents to the CDSCO office.
It is necessary to ensure that all the documents required for Cosmetics license are properly scanned, legible and in the correct format if you are submitting them online. Because the CDSCO portal does not approve the incomplete or improperly filled application and return it to the applicant. This will lead to delays in the process.
How Long Does the Cosmetics Import License Approval Process Take?
The processing time varies by country, but it typically takes 180 days to 6 months. If all the documents are correctly submitted then the process will be fast.
What Should I Do If My Application For Cosmetic Import License Rejected?
If your application is rejected, then you must review the feedback provided by the regulatory authority. Identify and correct errors in the documentation, and take help from experts to assist with reapplication.
How will ELT Assist You In Collecting Documents For a Cosmetic Import License?
At ELT Corporate, we simplify the complex process of cosmetic registration, ensuring a smooth journey to market. With our consultancy, you receive expert guidance in gathering all the essential documents, customized to meet the specific requirements of regulatory authorities. so that you can focus on bringing your products to the market with confidence.
Do I Need to Renew My Cosmetics Import License?
Yes, the cosmetics license needs to be renewed every three years to remain valid for the regulatory of the products.
Is a Certificate of Analysis (COA) required for each product?
Yes, a Certificate of Analysis (COA) is typically required for each product or batch. This document verifies the product’s compliance with safety and quality standards and is issued by a recognized testing laboratory.
Can I Apply For a Cosmetics Import License Online?
Yes, you can apply for the cosmetics import license online through the SURAM Portal by submitting the all required documents digitally.
What are the Consequences of Non-Compliance with Document Requirements?
Non-compliance can result in delays, fines, or the outright rejection of your import license application. In severe cases, non-compliant products may be seized or banned from the market.
Are There Penalties For Importing Cosmetics Without a License?
Yes, importing cosmetics without a license can cause fines, seizure of goods, and possible legal action.